
Read and carefully follow the Endologix AFX Endovascular AAA System Instructions for Use (IFU), which was revised in 2018 with updated information regarding Type III endoleaks.When making AAA treatment recommendations, and as part of the informed consent process, consider that recent data suggest there may be a higher than expected risk of Type III endoleaks in patients treated with any Endologix AFX endovascular grafts.The benefit-risk profile of AFX endovascular grafts compared to alternative treatment options should be considered for each individual patient.Prior to surgery, discuss the benefits and risks of all available AAA treatment options with your patients.Important Recommendations for Health Care Providers who treat and follow patients with an Endologix AFX Endovascular Graft System for Treatment of Abdominal Aortic Aneurysm: If you have an AFX endovascular graft and are overdue for a follow-up, make an appointment with the health care provider who treated your AAA or another vascular specialist to get your device checked.As a result the FDA recommends at least yearly, lifelong follow-up for all patients who have had their AAA treated with any AFX endovascular graft system.Be aware that recent data suggest there may be a higher than expected risk of blood continuing to leak into the AAA (Type III endoleak) which can result in serious injury, including death when any AFX endovascular graft is used for the treatment of AAA.If you have any type of Endologix AFX endovascular graft, contact the health care provider who treated your AAA or another vascular specialist about further care and to discuss continued follow-up.If you do not know if you have an AFX endovascular graft or if you do not have your implant card, please contact the health care provider who treated your AAA or the hospital where you were treated to find out. If you have already had treatment of your AAA with an endovascular graft system, review the implant card that you received at the time your AAA was treated to determine if you have any type of Endologix AFX endovascular graft implanted.Prior to surgery, discuss the benefits and risks of all available AAA treatment options with your health care provider.

Be aware that the FDA has approved endovascular grafts made by various manufacturers for the treatment of AAA, and each device has specific benefits and risks.Patients: Important Recommendations If You Have or Are Considering An Endologix AFX Endovascular Graft System for Treatment of Abdominal Aortic Aneurysm
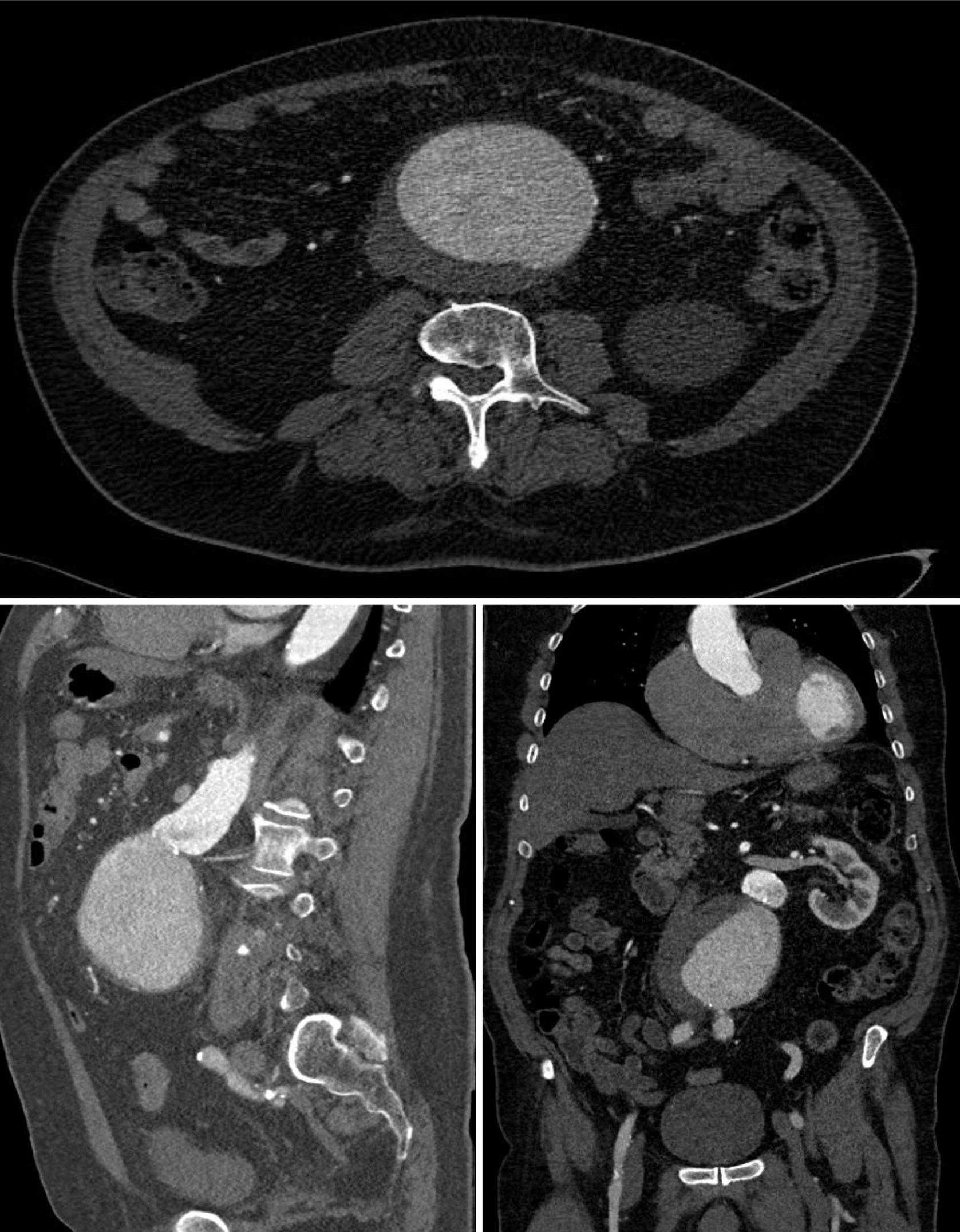
However, while we continue our evaluation, we are emphasizing the importance of at least yearly, lifelong follow-up for all patients who have any type of Endologix AFX endovascular graft in order to monitor for Type III endoleaks. We recommend lifelong follow-up for patients treated with any endovascular graft. It is important for patients and health care providers to be aware that data from an integrated healthcare system, published in a recent conference abstract, suggest there also may be a higher than expected risk of Type III endoleaks occurring with the use of AFX with Duraply and AFX2 endovascular grafts. We’ve previously communicated about the greater risk of Type III endoleaks occurring with the Endologix AFX with Strata device compared to other endovascular graft systems, which can result in serious injury. The FDA is evaluating new information about the risk of blood continuing to leak into the aneurysm (Type III endoleak) when Endologix AFX endovascular grafts (AFX with Strata, AFX with Duraply, or AFX2) are used for the treatment of abdominal aortic aneurysms (AAA).

DecemUpdate on Risk of Type III Endoleaks with Use of Endologix AFX Endovascular AAA Graft Systems: FDA Safety Communication.


 0 kommentar(er)
0 kommentar(er)
